|
I) Structure of matter A) Democritus – Greek philosopher 1) Matter could be divided and divided and divided until smallest unit is reached that cannot be divided further a) Atom 1) Smallest complete unit B) Combinations of matter 1) Compounds a) Two or more elements chemically combined 1) Molecule i) Smallest particle of any substance that can exist independently 2) Mixtures a) Combination of two or more substances physically combined b) Solution 1) A mixture in which substances are dissolved evenly throughout C) Properties of matter 1) Volume a) Volume (v) = length (l) X width (w) X height (h) b) cm3 2) Mass a) Amount of material 1) g or kg 3) Weight a) Measure of the pull of gravity 4) Density a) Mass of a substance contained in a given volume 1) density (d) = mass (m) ÷ volume (v) 2) g/cm3 D) Energy and changes in matter 1) Types of energy a) Potential energy 1) Stored energy b) Kinetic energy 1) Movement 2) Forms of energy a) Heat 1) Quantity of energy 2) Temperature i) Measure of the average kinetic energy of the molecules of a substance b) Chemical 1) Food 2) Fuel c) Nuclear 1) Nuclei of atoms d) Radiant 1) Travels through space by waves i) Radio ii) Microwaves iii) Infrared iv) Visible light v) Ultraviolet vi) X-rays vii) Gamma rays 2) Wavelength i) Distance from one peak of wave to the next |
Rutherford Model of the atom |
|
Calcium Fluorite (CaF2) |
|
|
Chromium (Cr) |
|
|
Structure inside an atom |
|
|
Potential Energy |
|
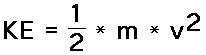 m = mass of object v = speed of object Kinetic Energy |
|
|
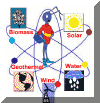
Renewable Energy |
|
|
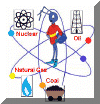
Non-renewable Energy |
|
|
Measuring Wavelengths |